Have you ever wondered how your body maintains its delicate internal balance, especially when it comes to acidity? Enter the unsung hero of our circulatory system: the carbonic acid-bicarbonate buffer. This remarkable chemical system plays a crucial role in keeping our blood pH stable, ensuring that our cells can function optimally. Let’s dive into this fascinating aspect of human physiology!
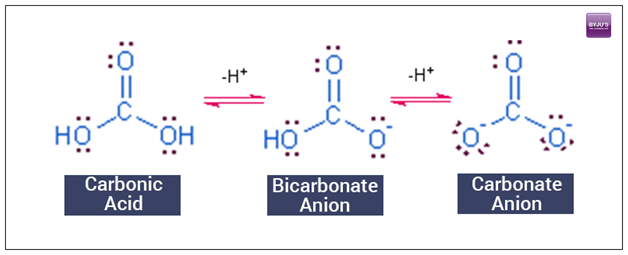
At its core, the carbonic acid buffer is a chemical balancing act between carbonic acid (H2CO3) and its salt form, bicarbonate (HCO3-). This dynamic duo works tirelessly to keep our blood pH within the narrow range of 7.35 to 7.45. Why is this so important? Even slight deviations from this range can lead to serious health issues, affecting everything from enzyme function to oxygen delivery.
The magic of this buffer system lies in its ability to neutralize both acids and bases. When excess acid enters the bloodstream, bicarbonate ions swoop in to neutralize it. Conversely, if the blood becomes too alkaline, carbonic acid steps up to the plate, releasing hydrogen ions to bring the pH back down.
But here’s where it gets really interesting: this buffer system doesn’t work alone. It’s intimately connected to our respiratory and renal systems. The lungs can expel excess carbon dioxide (which forms carbonic acid when dissolved in blood), while the kidneys can adjust the levels of bicarbonate. This intricate interplay allows for fine-tuned control of blood pH.
One of the most fascinating aspects of this system is its efficiency. Under normal conditions, there’s about 20 times more bicarbonate than carbonic acid in our blood. This ratio allows the buffer to handle a wide range of pH fluctuations, making it our body’s first line of defense against acid-base imbalances.
The complexity of the human body is crazy, and HSC Chemistry is a great way to understand it.
Jamyson Gouveros

